1 Battery decline and causes
With the rapid development of the communications industry, VRLAB is increasingly being used in remote rural areas and mountainous areas. Due to the large size and lack of professional knowledge of maintenance personnel, coupled with abnormal power supply, there are frequent power outages. This leads to different defects in the battery during use. In particular, batteries with deep discharge often have early battery failure. The main forms of battery failure are: corrosion deformation of the positive electrode plate, softening and falling off of the positive electrode active material, sulphation of the surface of the electrode plate or the production of lead velvet, internal crystal short circuit, and the like.
In order to achieve higher recombination efficiency, VRLAB is generally designed for lean electrolyte, that is, the amount of acid is used to control the capacity of the battery. This design works well from the theory or in the laboratory, but it often appears in the hands of users. Premature failure, especially in the case of frequent power outages, when the battery is over-discharged, the electro-hydraulic density drops below l.06/cm3, or even lower, causing a sharp increase in the concentration of free lead in the electrolyte, which is caused by The root cause of battery failure.
2 Factors affecting battery life
The battery is a kind of chemical power source. Its structure is similar. It is composed of a south positive electrode, a negative electrode, an electrolyte, a separator and a container. The active material of the negative pole and the electrolyte react chemically and play a leading role in generating current of the battery.
There are many factors that affect the actual service life of VRLAB. The main functions are as follows.
2.1 The effect of floating charge setting on battery life
The setting of the float voltage has a very important influence on the life of the battery. The unreasonable float voltage mainly affects the corrosion rate of the gate grid of the battery and the gas discharge in the battery.
2.2 The effect of balanced charging method on battery life
Balanced charging is sufficient to prevent some batteries from replenishing due to inconsistencies in capacity and terminal pressure. The amount of gas generated during equalization charging is several times higher than that during float charging, so the charging time should not be too long, and the charging voltage should not be too high, so as to avoid the surplus gas affecting the re-combination efficiency of oxygen, the amount of water loss increases, and the board is made. The gate corrosion rate increases, which can damage the battery.
2.3 Overdischarge
Excessive discharge of the battery is an important factor affecting the battery life. This situation mainly occurs after the power supply is cut off and the battery pack is in the period of power supply to the load. When the battery is over-discharged, a large amount of lead sulfate inside the battery is adsorbed to the surface of the cathode, and the "sulfated" lead sulfate forming the battery's rigid electrode itself is an insulator. The more lead sulfate formed by the cathode, the greater the internal resistance of the battery, the worse the charge and discharge of the battery, the faster the battery capacity drops, and the shorter the service life.
2.4 Influence of operating conditions on the life of valve-regulated lead-acid batteries
The operating conditions of the battery also have an important impact on the life of the battery. If used at high temperatures for a long period of time, the battery life is reduced by about half for every 10 °C increase in temperature.
Float operation is the best operating condition of the battery. The battery is always fully charged during operation. Under this condition, the battery will reach the longest service life.
The battery is frequently discharged for a long time and often undergoes deep discharge in the absence of full charge, so that the battery is in a deficient state for a long time, and the internal plates are vulcanized, resulting in a rapid decline in capacity and a backward battery.
3 activation repair of backward batteries
3.1 Battery internal reaction principle
PbSO4 in VRLAB electrolyte is always saturated. PbSO4 is a poorly soluble substance. The dissolution and precipitation of lead sulfate in the electrolyte are in equilibrium. Generally, the density of sulfuric acid at the beginning of battery discharge is 1.30g/cm3, and the mass percentage is 39.1%, with the increase of the depth of discharge, the mass percentage concentration decreased below 8.7%, the density was below 1.06g/cm3, and sometimes even lower, close to neutral.
Battery discharge reaction is 
It can be seen from the reaction formula that sulfuric acid not only conducts electric current, but also participates in an electrochemical reaction, and sulfuric acid is continuously reduced during discharge to form PbSO4 and water.
After the battery is discharged, if it is not charged in time or is not fully charged, the lead sulfate produced by the discharge will be crystallized into irreversible crystals of lead sulfate, causing the plate to be vulcanized and the battery to fall behind.
3.2 Activation of the battery
Battery charging reaction is
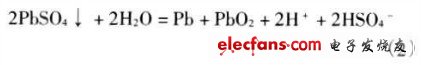
The charging and discharging process of the battery is to divide the pulse charging into one or several stages, and automatically charge according to the charging characteristic curve of the battery. The designed charging mode is “constant current→ (all charged geese value) constant pressure and reduced flow _ (automatic discrimination) Turned into) constant current discharge" three-band type to cool the electrolyte. This method is ideal and can eliminate vulcanization.
The battery is subjected to pulse charging and constant current discharge repeatedly cycle, and the internal lead sulfate crystal is activated to increase the density and mass concentration of sulfuric acid. With the deepening of the activation and repair, the density of the sulfuric acid of the battery reaches 1.30g/cm3, and the mass percentage The concentration reached 39.1%, and the dissolution and precipitation of lead sulfate in the electrolyte were in equilibrium. 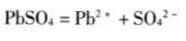 .
.
Obey the rules of solubility in solution, ie ![]() .
.
The battery is completely repaired and the battery life is extended by one to two cycles.
3.3 Treatment of severely backward batteries
For batteries with severe plate vulcanization, electrolyte dry or short circuit, open circuit (internal resistance is severely large, voltage is high or zero) should be replaced immediately. Short-circuit the battery to the 2V battery pack.
3.4 battery activation repair
Not all backward batteries can be repaired, resulting in a lot of battery lag factors, generally divided into 7 types, namely plate expansion, plate corrosion, plate passivation, effective material shedding, electrolyte dry, plate short circuit, pole The board is vulcanized. The first four are irreparable and the last three are repairable. Among them, the plate vulcanization leads to the largest proportion of battery backwardness, up to 90%. Therefore, for the capacity of 40%~80% of the rated capacity, the success rate of battery repair is relatively high. The test shows that the repair rate is over 95%. The success rate of backward battery repair is less than 40% of the rated capacity.
4 Charging settings for battery activation repair
Battery activation requires repeated discharges. When charging some backward batteries, sometimes it does not charge. Therefore, maintenance personnel charge by increasing the charging voltage. It is considered that the higher the charging voltage, the better the battery can be repaired. This is a misunderstanding. This is because the water decomposition reaction is 2H2O=O2↑+4H++4e- (3)
If the voltage is not set too high, the battery will be overcharged, and the result may be the following two cases.
1) The internal oxygen composite reaction cannot recombine the oxygen in time, which will cause a large amount of oxygen, which is released from the exhaust valve (and may even cause the battery to explode), causing the capacity to drop.
2) The reaction of the positive electrode is PbSo4+2H2O=Pb02+3H++HS04-+2e. At the anode of the battery, the lead alloy is directly in contact with the active lead dioxide, and simultaneously immersed in the sulfuric acid solution, each of which is established with the sulfuric acid solution. Different balanced electrode potentials. In the state of charging the battery. The positive electrode reacts due to oxygen evolution. Water is consumed, and H+ is increased, resulting in an increase in acidity near the positive electrode, accelerating corrosion of the plates, and even causing severe corrosion of the plates, which causes the battery to be scrapped, and the originally repairable battery becomes an unrepairable battery. For this reason, it is recommended that the voltage at the time of equalization generally does not exceed 2.35V.
PCB LED Module, Lights Source Pcb Modules, Ceiling Led Pbc Modules, Ac Led Linear Module
Shenzhen Isource lighting Co., Ltd , https://www.isourceled.com